N-0053 General Lab Supplies
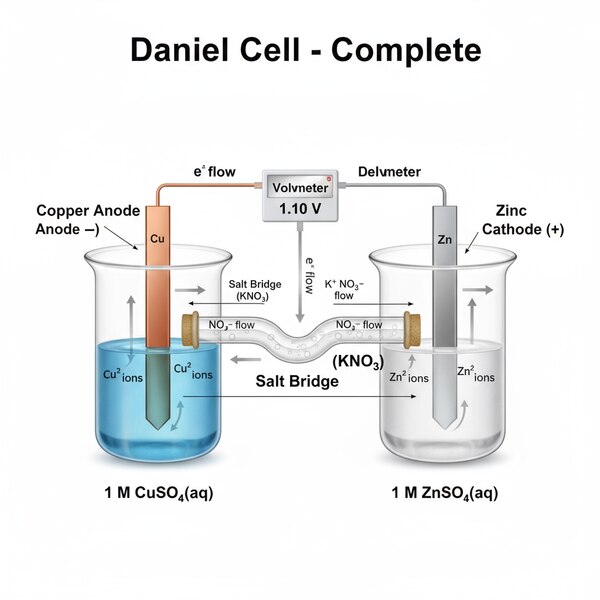
Carbon Electrode Rods with terminals, approximately 6 inches in length, are specialized rods primarily used as electrodes in laboratory and industrial applications. These rods are typically cylindrical and made of high-purity carbon or graphite, known for excellent electrical conductivity and heat resistance.
Description and Features:
Constructed from pure carbon or graphite material with high density and conductivity, ideal for efficient current flow.
Equipped with metal terminals (often brass or copper) at one or both ends, facilitating secure electrical connections to power sources or devices.
Approximately 6 inches in length (around 150mm), suitable for use in laboratory experiments, electrolysis, arc welding, and industrial processes requiring stable and durable electrodes.
The rods are resistant to high temperatures and chemical reactions, exhibiting durability under harsh electrical and thermal conditions.
Commonly used for arc welding applications, electrochemical cells, and as electrodes in various scientific and manufacturing processes.
The terminal fittings ensure efficient electrical contact, reduce resistance losses, and provide mechanical stability.
These carbon electrode rods with terminals offer reliable performance, longevity, and ease of use in electrical and thermal applications where stable, conductive electrodes are essential
Send
Message